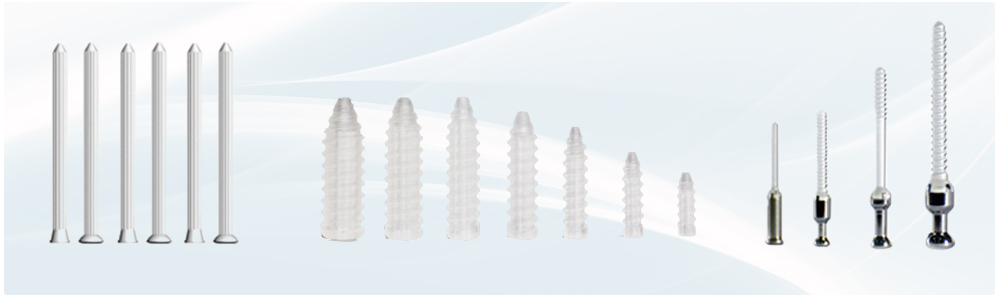
Bioretec products have their roots in biomaterials and implant technology. Now Bioretec’s R&D team, has created the fourth generation bioabsorbable products, offering new and advanced properties combined with clear benefits for surgeons and patients.
“Bioretec pins & screws” are manufactured from Bio-absorbable lactic / glycolic acid copolymer (PLGA) by M/s. Bioretec OY, Finland. The Bioretec products have been cleared by US FDA and the CE mark has been granted by KEMA. In addition, the products have been cleared for sales in several other countries worldwide.
Activa Pin and Activa Screw is delivered inside the Activa Pin / Activa Screw HOLDER. It is made to protect the pin / screw during storage/ delivery and for easy and aseptic implantation of the pin / screw.
ActivaPin™
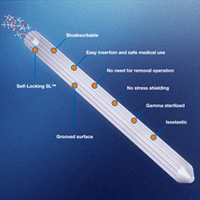 The ActivaPin™ bio-absorbable pins are constructed of bio-absorbable lactic/glycolic acid copolymer (PLGA). These polymers have a long history of safe medical use and they degrade in vivo by hydrolysis into alpha-hydroxy acids that are metabolized by the body.
The ActivaPin™ bio-absorbable pins are constructed of bio-absorbable lactic/glycolic acid copolymer (PLGA). These polymers have a long history of safe medical use and they degrade in vivo by hydrolysis into alpha-hydroxy acids that are metabolized by the body.
The ActivaPin™, belonging to the Activa family of products, is a super-strong pin suitable for many different indications. The product is the result of long-term collaborative development work carried out by surgeons and scientists. The experience contributed by the surgeons led the product development in the right direction, making the fourth-generation Activa products the best on the market.
Features:
1. High Strength offers stabilized fixation.
 2. Easy insertion.
2. Easy insertion.
3. Safe medical use.
4. The bending modulus is close to value of cortical bone.
5. Designed to gradually restore original load carrying capacity of bones.
6. Packaging, instrument & implant design also enables aseptic surgical procedure.
7. These feature self-locking SL technology with patented grooved surface design.
ActivaNail™
 The Activa Nail™ Flat and Activa Nail™ Conical bio-absorbable nails are constructed of bio-absorbable lactic/glycolic acid copolymer (PLGA). These polymers have a long history of safe medical use and they degrade in vivo by hydrolysis into alpha-hydroxy acids that are metabolized by the body. The manufacturing process generates the high initial mechanical strength and stiffness of the Nails. Properly used, in the presence of adequate immobilization, the Activa Nail™ maintains accurate alignment of small bone fractures, apical fragments and osteochondral fractures after surgical procedure. As the operated bone fracture or osteotomy gains strength during healing, the Activa Nail™ gradually loses its strength, however, maintaining its function at least 8 weeks. Bioabsorption takes place approximately within two years thus eliminating the need for implant removal surgery.
The Activa Nail™ Flat and Activa Nail™ Conical bio-absorbable nails are constructed of bio-absorbable lactic/glycolic acid copolymer (PLGA). These polymers have a long history of safe medical use and they degrade in vivo by hydrolysis into alpha-hydroxy acids that are metabolized by the body. The manufacturing process generates the high initial mechanical strength and stiffness of the Nails. Properly used, in the presence of adequate immobilization, the Activa Nail™ maintains accurate alignment of small bone fractures, apical fragments and osteochondral fractures after surgical procedure. As the operated bone fracture or osteotomy gains strength during healing, the Activa Nail™ gradually loses its strength, however, maintaining its function at least 8 weeks. Bioabsorption takes place approximately within two years thus eliminating the need for implant removal surgery.
Features:
1. Improved fixation stability with flake fractures.
2. Self-Locking SL.
3. Grooved surface design.
4. High Strength properties offer easy insertion & safe medical use.
5. No stress shielding.
6. No need for removal operation.
7. Implant is supplied Gamma sterilized.
8. Two different head designs; Conical and Flat.
ActivaScrew™ - Bioabsorbable Implants
 Bioretec`s product concept Bioretec has developed a product concept that allows for the aseptic use of a bio-absorbable product in the operating theatre. The concept facilitates and speeds up the surgeon’s work while also optimizing safety. The Activa Screw™ is delivered with an AO-compatible single-use adapter in a specifically designed holder that permits the aseptic use of the product and offers a safe and user-friendly way to attach the screw to an instrument and transport it to the operating target.
Bioretec`s product concept Bioretec has developed a product concept that allows for the aseptic use of a bio-absorbable product in the operating theatre. The concept facilitates and speeds up the surgeon’s work while also optimizing safety. The Activa Screw™ is delivered with an AO-compatible single-use adapter in a specifically designed holder that permits the aseptic use of the product and offers a safe and user-friendly way to attach the screw to an instrument and transport it to the operating target.
Bioretec’s Activa Screw™ is a fourth-generation (PLGA) bio-absorbable implant. Its properties are unique and proven to be top-quality. Using the Activa Screw™ allows surgeons to attend to more patients, as removal surgery is no longer needed and time is saved during the operation itself by the advanced product technology.
Features:
 1. Full compatibility with AO-instrumentation. No need for customized instrumentation!.
1. Full compatibility with AO-instrumentation. No need for customized instrumentation!.
2. High Strength properties offers stabilized fixation, easy insertion and safe medical use.
3. The bending modulus is closer the total value of cortical bone compared to metallic implants.
4. It’s designed to gradually restore the original load carrying capacity of the bone.
5. Bio-absorbable eliminates the risk of long term complications and removal operations.
6. Packaging, instrument and implant design enables aseptic surgical procedure.
7. Auto-Compression™ technology with its patented mechanical activity.
ActivaScrew™ Cannulated
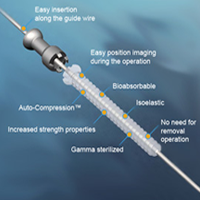
The Activa Screw™ Cannulated produces first in the world Auto-Compression™ and enables aseptic handling throughout the surgery.
The Activa Screw™ Cannulated also offers a wide range of lengths that gives surgeons more choices and possibilities for surgical operations, and it is offered in three different diameters: 3.5 mm, 4.0 mm and 4.5 mm.
Alternatively, when increased strength properties are preferred, 4.0 mm and 4.5 mm Activa Screw™ Cannulated can be used applying combination technique with 1.5 mm ActivaPin™ by inserting the pin inside the ActivaScrew™ Cannulated.
The ActivaScrew™ Cannulated bio-absorbable screws are constructed of bio-absorbable poly (L-lactide-co-glycolide) (PLGA).
Properly used, in the presence of adequate immobilization, ActivaScrew™ Cannulated maintains accurate alignment of bone fractures, osteotomies, arthrodeses, bone grafts and osteochondral fractures after surgical procedure. As the operated bone fracture or osteotomy gains strength during healing, the ActivaScrew™ Cannulated gradually loses its strength, however, maintaining its function at least 8 weeks. Bioabsorption takes place approximately within two years thus eliminating the need for implant removal surgery.
Features:
- Easy insertion along the guide wire
- High Strength offers stabilized fixation
- Designed to gradually restore the original load carrying capacity of the bone
- Bio-absorbable - No risk of long term complications. No removal operations
- Packaging, instrument and implant design enables aseptic surgical procedure
- Patented Auto-Compression™ technology brings mechanical activity
ActivaScrew™ Interference

- ActivaScrew™ Interference is indicated for fixation of tissue, including ligament or tendon to bone, or a bone-tendon to bone in the presence of appropriate immobilization or controlled mobilization. Interference fixation is appropriate for surgeries of the knee, shoulder, elbow, ankle, foot and hand/wrist.
- ActivaScrew™ Interference is available as fully threaded screws in different sizes: diameters 4 - 10 mm and lengths
10 - 33 mm - ActivaScrew™ Interference is cannulated. It allows insertion of the screw over a guide wire (1.6 or 1.1 mm) easing the screw placement
- Center driven Torx driver extends the length of the ActivaScrew™ Interference transmitting force throughout the screw making insertion optimal.
- ActivaScrew™ Interference is cannulated enabling the use of guide wire during the operation. It helps the position imaging during the operation.
- High initial mechanical properties and isoelasticity of ActivaScrew™ Interference are created during the manufacturing of the product. These features increase the reliability of the screw in clinical use
- After operation ActivaScrew™ Interference slightly contracts in length and increases in diameter due to material memory effect improving screw’s fit inside the tunnel and thus reducing the risk of unstable fixation. Self-Locking SL™ property is activated under hydrolytic conditions.
- The bending modulus of ActivaScrew™ Interference is closer to the value of cortical bone compared to metallic implants
- Bioabsorption takes place approximately within two years thus eliminating the need for implant removal surgery
- ActivaScrew™ Interference is made of bioabsorbable polymer. It enables drilling through the product in the need of re-operation. / It enables overdrilling in the need of re-operation.
- Metallic implants are known to disturb MRI imaging. Bioabsorbable materials are not magnetic and do not disturb MRI imaging.
Implant is supplied Gamma sterilized - safe, free of gas remnants, reduced cross infection risk
CiproScrew™
 There are several factors that may increase the risk of implant related infections in patients undergoing surgery. Such factors are e.g. smoking, diabetes, high age and open contaminated fractures for example in case of trauma. On average a few percent of initially inserted fixation devices become contaminated and cause bacterial infection. Nevertheless, in case of open fracture the incidence of infection is reported to be as high as 30%. The treatment of implant-related infections is very challenging, long-lasting and expensive. Most common treatments include long term antibiotic medication and removal of all foreign bodies. By introducing the world`s first antibiotic releasing bio-absorbable screw – CiproScrew™ Bioretec offers solution to prevent implant related infection.
There are several factors that may increase the risk of implant related infections in patients undergoing surgery. Such factors are e.g. smoking, diabetes, high age and open contaminated fractures for example in case of trauma. On average a few percent of initially inserted fixation devices become contaminated and cause bacterial infection. Nevertheless, in case of open fracture the incidence of infection is reported to be as high as 30%. The treatment of implant-related infections is very challenging, long-lasting and expensive. Most common treatments include long term antibiotic medication and removal of all foreign bodies. By introducing the world`s first antibiotic releasing bio-absorbable screw – CiproScrew™ Bioretec offers solution to prevent implant related infection.
Features:
1. Inhibition of ciprofloxacin sensitive organisms to attach the surface of the screw.
2. An adequate range of fully threaded and partially threaded screws.
 3. High usability for various indication.
3. High usability for various indication.
4. Compatibility with AO-instrumentation.
5. Auto-Compression™, reduces the risk of unstable fixation.
6. Isoelasticity; the bending modulus is closer to the value of cortical bone compared to metallic implants.
7. High strength properties offer easy insertion and safe medical use.
8. No stress shielding.
9. No need for removal operation.
10. Bio-absorbable implants transfer gradually stresses to the bone, thus reducing potential stress shielding.
11. Implant is supplied Gamma sterilized – safe, free of gas remnants, reduced cross infection risk.